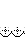- Last week review...
 Result
ResultPicture 1

This week I am going to share on how to get a recombinant Bacmid and how to package this recombinant Bacmid into a virus.
This will require 2 steps, Transformation into a special cell call 10a and Transfection into host cells respectively.
Method is just some common protocol, whats more impt is the principle =)
Transformation
Method
1. Obtain the 10a competent E.coli cells from -80ºC freezer (1 vial = 100μl) and carry it in ice box. Thaw the cells on ice.
2. Aliquot the appropriate amount of recombinant plasmid DNA into an eppendorf tube.
3. Place the tube onto ice.
4. Aliquot 25μl of 10a competent E.coli cells into the tube ON ICE, and incubate for ½ hour.
5. Meanwhile, check that antibiotic plates (Kanamycin, Getamycin, Tetracycline, Bluo-gal, IPTG) are warmed up in the 37°C incubator (plates are inverted to prevent contamination) and ensure that water bath is heated up to 42°C
6. After the incubation for ½ hour, heat shock the bacteria cells for exactly 45 seconds in 42°C water bath without shaking.
7. Immediately transfer it onto ice for 1 to 2 mins
8. In the LAF hood, add S.O.C medium at room temperature (similar to LB medium) in 1:9 ratio to competent cells. è therefore add 225μl of S.O.C medium into each tube.
9. Shake the tube at 37°C at 300rpm in an incubator for 4 hour.
10. Obtained the transformed cells from the incubator and place it in the LAF hood.
11. Using a sterile disposable spreader, plate the whole volume (250 µl) of the each tube of cells onto the pre-warmed antibiotic plate.
12. Allow both plates to stand in the LAF hood with covers fully covered for 1 to 2 mins to allow E.coli cells to attach to agar.
13. Invert the plates (to prevent contamination) and put it into the 37ºC incubator. (incubate for 24 hours to see colonies and 48hours to see blue and white colonies)
14. After 48 hours, obtain the plate from the incubator, parafilm it and store in 4ºC fridge.
Principle
The gene of interest in the recombinant plasmid is flanked by the left and right arms of Tn7 and contains ampicillin and gentamycin resistance gene for selection, and other features to form a mini Tn7. (Shown in picture 1-above)
Host cell for this plasmid, 10a cells, contains a bacmid with a mini-attTn7 target site and a helper plasmid. When recombinant combinant plasmid was transformed into the 10a cells, transposition between the mini Tn7 on the plasmid and mini-attTn7 target site on the Bacmid occurred to produce a recombinant Bacmid. (transposition is something like “transfer” the recombinant plasmid into the Bacmid).

 Recombinant bacmid was selected out on specific antibiotics plates containing (tetracycline, gentamycin, kanamycin, IPTG and bluo-gal), restreaked on new antibiotic plates, PCR and analyzed, followed by extraction and purification using the Miniprep kit.
Recombinant bacmid was selected out on specific antibiotics plates containing (tetracycline, gentamycin, kanamycin, IPTG and bluo-gal), restreaked on new antibiotic plates, PCR and analyzed, followed by extraction and purification using the Miniprep kit.Tetracycline – because of tetracycline gene in the helper plasmid
Gentamycin- because of gentamycin gene found in recombinant plasmid
Kanamycin- because of kanamycin gene found int the Bacmid
IPTG & bluo-gal- For blue-white selection. Presence of recombinant plasmid in bacmid (white). No recombinant plasmid in bacmid (Blue).
 Transfection
TransfectionMethod
*transfection can cause alot of cells to die, as the DNA:lipid complexes complex that are to be taken up by the cells are toxic, therefore to ensure we get as much transfected cells as possible we have to be very careful and ensure the conditions are optimal.
1. In a 6-well culture plate, seed 4 x 105 host cells/ml per well in 2ml of supplemented medium (medium suitable for culturing the host cells)
2. Allow the cells to attach at 27°C for at least 1 hour.
3. After about 15 mins later, obtain the unsupplemented medium (free of serum) from the fridge and a new 50ml tube and place it in the BSC.
4. In the BSC, aliquot about 12ml of unsupplemented medium into a new sterile 50ml tube, and return the original bottle back into the fridge.
5. We have to prepare 2 eppendorf tubes, 1 eppedorf tube for the cellfectin reagent and the other for the recombinant Bacmid DNA.
6. In the BSC, prepare as follows in 1.5ml eppendorf tubes:
Tube 1: Dilute 1µg (~0.7µl of our sample) of each purified bacmid DNA in 100µl of unsupplemented medium. (Important: ONLY pipette 1 time)
Tube 2: Mix cellfectin® reagent thoroughly before use by inverting the main tube 5-10 times. Remove 6µl of cellfectin® reagent and dilute in 100µl of unsupplemented medium.
Tube 3: Combine 100µl of the diluted Cellfectin® Reagent to tube of sample ( ~210µl). Mix gently by just tapping the bottom of the bottle, and incubate for 30minutes at RT in the BSC.
7. By now the cells should have incubated for about ½ hour. Obtain the cells on the 6 well plate from the incubator and check under the phase contrast microscope that the cells has already attached to the surface of the 6 well plate.
8. While the DNA:lipid complexes are incubating, remove the medium from the cells in the 6 well plate and wash once with 2ml of unsupplemented medium. Do this by pipetting away the old medium and replace it with 2ml of
unsupplemented medium and swirl the plate to make sure that the cells are washed. Then return the plate into the incubator.
9. After the ½ hour incubation of the Bacmid DNA: Cellfectin® Reagent complex in the BSC, take out the 6 well plate from the incubator and introduce it into the BSC.
10. Pipette out the 2ml of unsupplemented medium and add 0.8ml of unsupplemented medium to each well, followed by 200µl of the Bacmid DNA: Cellfectin® Reagent complex, swirl and rock the 6 well plate after adding.
11. Label the wells according to what was added and incubate in the 27°C incubator for 5 hours.
After about 4 hours of incubation, prepare unsupplemented medium containing antibioticsà 50units/ml penicillin and 50µg/ml streptomycin final concentration
12. After 5 hours, remove the Bacmid DNA: Cellfectin® Reagent complex from the well and add 2ml of supplemented medium to cells.
13. Incubate the transfected cells in the 27°C incubator for 72 hours or until we start to see signs of viral infections.
Principle
Purified recombinant bacmid were complex with cellfectin® reagent and transfected into host cells. (how cellfectin reagent work is already shared in week 1). This complex will cause formation of liposomal structure that causes host cells to uptake the complex.
The original bacmid before transformation was done, is actually a modified genome of the virus (got lac Z and antibiotic genes etc). The recombinant bacmid is the genome of the virus, now consisting of the recombinant plasmid in its Tn7 site.

 After about 4 days, signs of viral infection start to appear, and viruses start to bud out.
After about 4 days, signs of viral infection start to appear, and viruses start to bud out.Signs of infect:
1. Black dots in cell nucleus
2. Cells look swollen
3. Rough surfaces of cells
4. So cells will look fused together
 Preparation of the supplemented medium containing antibiotics
Preparation of the supplemented medium containing antibioticsThe purpose of adding antibiotics to this medium that the cells are going to be incubated in for 72 hours is to prevent bacteria contamination. Bacmid is a piece of very large DNA, and since it is left as a DNA in the naked form to be incubated with the cells, it can easily be contaminated by Bacteria, to prevent this from happening we added this 2 antibiotic as recommended by the manufacturers.
we usually do about 200 folds of dilution
Therefore, since in total we prepare about 40ml of the supplemented medium in this case, and antibiotic mixture (200µl) and pipette up and down to mix thoroughly.
Supplemented and unsupplemented medium
For supplemented medium, we have to add supplement such as FBS (Fetal Bovine Serum). Serum is expensive and also needs to be purified from the medium if the DNA is to be used for transfection, as the serum can interfere with transfection and results in lower efficiency. But serum allows the cells to grow better as it provide more nutrient than unsupplemented medium. Unsupplemented medium is only use for transfection.
Viruses are collected in an eppendorf tube and stored at 4 degree celcius fridge. These viruses are amplified to produce second and third batch of viruses to be used (will be shared in next post). To check for the titer of the viruses constructed, plaque assays are done. (shared in post 1)
 Thanks for reading this very long post n not v nice pics i noe.. hahas..cos its all molecular stuff, i cant really show u guys the real thing =/ Hope u all understand what i have shared.
Thanks for reading this very long post n not v nice pics i noe.. hahas..cos its all molecular stuff, i cant really show u guys the real thing =/ Hope u all understand what i have shared.Feel free to ask qns. Enjoy ur attachment =)
Jean Leong
TG02